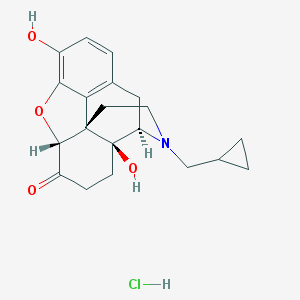
Naltrexone
Low Dose Naltrexone is widely touted on the Internet almost as a wonder drug . But because it is out of patent there is little incentive for drug companies to explore its use in low doses. Also because of the lack of peer reviewed double blind studies of naltrexone used in low doses, most doctors don’t prescribe it.
In November 20, 2000, a New York physician, Bernard Bihari, MD filed a patent on a way to treat prostate cancer with low dose naltrexone, using a dosage level in the range of 1.0 mg. to 10 mg. per day. Patent number 6,384,044 was awarded to Dr.Bihari on May 7, 2002. You can see the patent HERE
Since Dr.Bihari’s work on prostate cancer, people have been trying low dose naltrexone for conditions such as Crohn’s disease, rheumatoid arthritis, fibromyalgia, pain of endometriosis, various cancers, and assorted autoimmune diseases.
What is Naltrexone?
Naltrexone is supplied as naltrexone hydrocloride for oral use in 50 milligram tablets. It is an opioid receptor antagonist used primarily in the management of alcohol dependence and opioid dependence.
how to compound low dose naltrexone
Naltrexone and its metabolite 6-beta-naltrexol reverses the effects of opioids by binding to various opioid receptors in the central nervous system, including the mu-, kappa- and gamma-opioid receptors. A 50 mg. dose once per day inhibits the opioid effects of analgesia, euphoria, sedation, respiratory depression, miosis, bradycardia, and physical dependence. Naltrexone is longer-acting and more potent than naloxone.
Low dose naltrexone is used off-label for many different conditions unrelated to alcohol or narcotic dependence. None has received FDA approval and most physicians are wary of using it in non-approved low doses, although they are legally able to do so. Patients who use it do so because they have conditions that are difficult to treat with conventional drugs and they go to great efforts to find doctors to help them experiment.
Naltrexone Solubility and Stability
Naltrexone is highly soluble in water – 100 mg/mL (as the hydrochloride salt) – making it very easy to dissolve in water to prepare small doses. Fifty milligrams (mg) dissolved in 50 milliliters (ml) of water makes a solution containing one mg per ml. The usual low dose is between 3-4.5 mg.
How to Compound Low Dose Naltrexone
It is also stable in water solution for at least 60 days when refrigerated. Dissolving a tablet in distilled water yields a solution that is slightly cloudy due to the excipients in the tablet – small amounts of non-active binders and coloring: colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, synthetic red iron oxide, synthetic yellow iron oxide and titanium dioxide.
Clinical Trials
Naltrexone HCL by itself is no longer covered by patents. Limited patent protection makes it difficult to fund clinical trials. Because there are few clinical trials, the FDA has not approved its use for off label applications. Without FDA approval, drug manufacturers cannot market the product for any purpose other that that receiving the initial approval. Naltrexone is widely used to treat opioid addiction.
A time release formulation of Naltrexone is covered by a patents assigned to Orexigen Therapeutics, Inc. and is part of the patent protection for Conntrave extended-release tablets, a weight loss drug. It contains a low dose ( 8mg) of naltrexone hydrochloride and 80 mg of bupropion hydrochloride, an antidepressant drug. The combination is covered by patent # 9,119,850.
How to get Low Dose Naltrexone
Nevertheless there are a few small clinical trials in the works. The website lowdosenaltrexone.org chronicles the use and popularity of low dose naltrexone. HERE